cadaver bone graft rejection symptoms
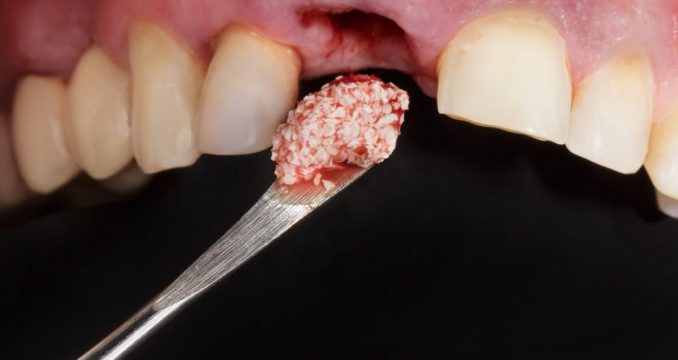 Bone Graft Dental Rejection Symptoms
Bone Graft Dental Rejection SymptomsWarning: The NCBI website requires JavaScript to operate. Adverse reactions and events related to musculoskeletal theft: revised by the NOIFYM World Health Organization Project. Instituto Nacional de Medicina de la Salud, Universidad de Washington, D.E.U., Universidad de la Universidad de Brussels, 808 route from Lennik, 1070 Brussels, Belgium L. Muylle2Antwerp, University of Edegem and Antwerp, University of the University of Rome, Antwerp, Belgium The use of allografts of bone and connective tissues has increased rapidly and has exceeded the use of autografinjerin. Being of human origin, bone and tendon attachments pose the risk of disease transmission and complications have been reported. As part of the NEWS Project led by the World Health Organization, an effort was initiated to improve the recognition, reporting, monitoring and investigation of the adverse results of allografts, achieving a comprehensive review of disease-related transmission and failures. Those who imply the use of musculoskeletal attachments are reported here. An important objective is to involve orthopedic surgeons in improving the safe use of musculoskeletal allografts. MethodsWe review medical literature, request reports from surgeons in certain professional organizations and organizations of informally surveyed tissue banks and selected professionals from tissue banks to discover reported and unreported cases of adverse results. We analyze each case to decide the probability that the complication was really related to allograft. ResultsThe efficiency of the procedures involved in bone banking and tendon alograft has improved significantly over the last three decades. The evolution of the incidence of reactions and adverse events reported positively reflects the safety of transplanted tissues. Cases of bacterial and viral transmission by bone grafts and tendons occurred mainly with those containing viable cells, were not processed to remove cells, or were not disinfected or sterilized. We have documented cases of transmission of human immunodeficiency virus (HIV), hepatitis C virus (HCV), human T-lymphotropic virus (HV), unspecified hepatitis, tuberculosis and other bacteria. Reporting on these adverse results has resulted in remedial action and has significantly improved the security of the use of the approval. However, it is likely that not all cases have been reported and investigated. Conclusions Taking into account the high quality standards achieved in many countries, the best approach to further improve the safety of allografts is through a systematic reporting of all serious reactions and adverse events in the context of a global bio surveillance programme. IntroductionThe grafts of individual alogrations and tendons, including the demineralized bone matrix, represent a useful and sometimes indispensable tool for the orthopedic surgeon to repair bone defects and improve the function. Even if in the cases reported previously of viral transmission (the last occurred in 2000) the non-sensitivity virus of hepatitis C (HV) or the human immunodeficiency virus (HIV) were made prior to the use of the graft, a major concern of its use remains the well-documented risk of transmitting infectious agents and malignant cells []. Notification of complications associated with allograft by the orthopaedic surgeon triggers an investigation that is essential to correct errors and accidents and improve the safety of the use of alograft and to prevent bone donations from the same donor or from the same lot that is transplanted to other receptors. In the face of the globalization of tissue transplant, this requires systematic and rigorous bio surveillance procedures. As requested in World Health Assembly resolution 63.22 in 2010, the World Health Organization (WHO) in collaboration with the Italian National Transplantation Centre (CNT) and the European Union-funded project on monitoring and monitoring of human origin substances (SOHO V pestS) launched the NOTIFY Project to optimize global surveillance and monitoring for cell transplantation, tissue and organ. A key objective is to improve the recognition, reporting, monitoring and research of the adverse results associated with the transplant of tissues and cells [, ].The participants of the NEWS Project undertook a review of the adverse results of the bone transplant and allograft tendons, procedures followed by tissue banks to prevent the transmission of diseases, issues related to the recognition, notification and investigation of adverse results, and measures taken to improve the safety of allografts. Here we present a preliminary report of the work of the musculoskeletal group NEWS Project. MethodProject NOTIFY developed a database of adverse reactions and events related to the transplant of musculoskeletal alogramos collected from cases reported in the literature and specialists of tissue banks, orthopedic surgeons and members of the International Society of Orthopedic Surgery and Traumatology (SICOT). Members were consulted by e-mail and were asked to return a standardized questionnaire. The details of each case were reviewed to determine whether the transmission was confirmed by a bone or a allograft tendon. The risk associated with tissue transplantation was reviewed on the basis of the collected information. The following definitions of the adverse results of allograft adopted in the European Directives of Tissues and Cells were considered appropriate and useful for the international application with a slight modification to include "human application" and "donors":Averse event (SAE): any uncomfortable occurrence associated with the purchase test, processing, storage, "human distribution" of the tissues and cells that could lead to adverse transmission of disease ResultsTransmission of HIVNew cases of HIV infections reported are due to the use of frozen bones and/or tendons. In a brief report that did not describe details, a possible case was associated with the use of iophilised bone chips and in two possible cases, the nature of the bone was not known (Table). Except in a case where an uncertified donor, in 1996, all other cases occurred between 1984 and 1986, suggesting a beneficial effect of stronger donor selection procedures and a better effectiveness of HIV infection testing. Nine of the cases were due to the lack of HIV testing at the time of the acquisition of allografts and, in one case, due to the lack of HIV testing of the donor. The diagnosis was due to a positive test in seven asymptomatic patients and in five patients after the occurrence of general inflammatory symptoms. In eight cases the infection was confirmed in the donor, while in four cases the infection was not documented in the donor (two donors). The diagnosis was made three weeks to 1.5 years after the transplant. Two of the infected donors were multi-organic donors (one untested for antiHIV and another false antiHIV negative). Table 1 HIV transmission case Type of allograft Publication date ProvenAuthor (reference)1Frozen femoral heada19841988+CDC []4Cryopreserv bonea19841996+Schratt et al. []2Frozen femoral headb19851992+ Simonds et al. []3Bone chip, lyophilised bone allografts (not specified)a19851997+/−Karcher []1Frozen patella (including patellar ligament and tendon and a tibia section)b19861992+ Simonds et al. []1Frozen femoral heada19962001+/−Li et al. []aNo HIV testing was performed in anti-HIV-1 negative donorb, NAT positive Of 1 of these donors, the unprocessed cryopermerated bone was transplanted in 12 patients []. Four of these seroconverted patients, while seven are not. A patient died a natural death before performing the tests. From the other multi-organic donor, allografts were transplanted in 48 identified patients and 41 out of 48 patients were available for HIV testing []. The four organs transplant patients and the three newly frozen bone receptors without processing or seroconvert tendon, while 34 receptors of other tissues (two corneas, three liophilic soft tissues, 25 bones treated with ethanol, three irradiated hard matt with gamma and a freshly frozen bone cleaned to remove the bone marrow) would not seroconvert. Today, the risk of transmission of HIV to tissue receptors is significantly reduced by the use of NT tests. With the frequent addition of detection of p24 antigen followed by the use of HIV nucleic acid amplification tests (NAT), no new cases of HIV contamination have been reported over the past 15 years, although the performance of these tests is not a requirement in the EU. HCV Transmission All ten cases of HV transmissions were derived from the frozen or cryoperated alograft transplant that were not processed or sterilized strongly (Table ). The last reported cases were caused by tissue recovered in 2000. No cases have been reported since HCV NATs have been applied to donors by authorities in many countries, even if this review is not necessary. Diagnosis in infected patients was performed after the occurrence of acute clinical symptoms (one case) or after the back tests with new more sensitive tests of the involved donors. Table 2 Reported cases of HCV transmission Type of allograftDatePublication ProvenAuthor (reference)1Frozen bonea1986-19901993? Pereira et al. []1Frozen bonea19901992+Eggen and Nordbø []1Frozen bonea19911995+ Conrad et al. []3Cryopreserved soft tissue (fascia, ligaments)a1991+ Conrad et al. []3Frozen bone-tendon-boneb20002005+ Tugwell et al. []1Cryopreserv tendonb20002005+ Tugwell et al. []aNo testb anti-HCV Anti-HCV negative, no HCV NATEl role of Tugwell et al. [] illustrates the importance of rapid notification of suspected cases of virus transmission (bio surveillance) and full traceability of donor to patient and vice versa. A patient developed acute symptomatic hepatitis C six weeks after receiving a tendon patellar allograft. The treatment doctor informed public health officials of the infection. The later look showed that three receptors developed hepatitis C more than a year earlier, but these infections were not reported. If reported at that time, the use of 24 tissues from the same donor and at least three new HCV transmissions could have been prevented. In addition, it was not possible to locate two of the recipients. Forty allografts had been distributed in a period of 22 months of the same initially negative donor for HCV antibodies tested by a second-generation immunassay, but subsequently tested positive by HCV NAT after the report of an infected receiver. Of the 40 tissue receptors, three of the three receptors of allograft tendons and one of the three receptors of allograft tendons were deconverted (more than three organ receptors and one of the two vein receptors). In total eight seroconverted receptors and 30 no. As for the remaining ten, five receptors were already infected with HCV before implantation, but for the other five, no post-transplant serum was available. Hepatitis transmission, unspecified type For musculoskeletal allografts, a historical case of hepatitis has been reported not specified after the use of allograft bone. This case involved a frozen allograft in 1954 (Table). The receiver developed jaundice ten weeks after allograft implantation. Table 3 Non-specific hepatitis transmission type of alograftDatePublicationProvenAuthor1Hueso frozen19521954+/−Shutkin [] HTLV-1 Transmission A reactive test in an asymptomatic receptor was reported after the implantation of a frozen femoral head allograft of a patient who had previously been infected with the T-lymphotropic type 1 (HTLV-1) human virus through a contaminated blood transfusion (Table ). Table 4Reported case of HTLV-1nType of allograftPublicationProvenAuthor1Frozen femoral head1991+Sanzén and Carlsson []EBV, CMV, WNV and CJD transmission The transmission of Epstein-Barr virus (EBV), the cytomegalovirus (CMV), the virus of the West Nile (WN) With a vein, valve, skin or nervous allograft, viral transmission occurred only with fresh, frozen or cryopened alograft. The cases of CJD were only observed after the transplant of last mater [, , , , , , ] and alografts corneal [, ]. Although the transmission of the disease has not been observed prion by bovine xenografts, the risk is unknown and it is recommended to avoid the use of bovine xenografts. WHO recommends that allografts be exposed to 1 mol/l NaOH for one hour to prevent the transmission of prions, which is supported by most authors [, ].Bacterial infection transmissionBacterial infections resulting from the implantation of musculoskeletal allografts have been reported since 1953 in relatively limited numbers []. Of the revised literature cited in references, 49 possible transmissions were documented with only ten checks and 39 remaining possible [, , , , , , , , , , , , , ]. Symptoms of infection appeared between three days and 20 weeks after implantation. There is no specific trend from the identified bacteria, as they include species from all major groups (Table). Alografts are often used to repair bone defects or damaged tendons in patients who have had multiple operations, have altered vascularization on the operating site and are at greater risk of local infection. To be tested, contamination must be confirmed by the identification of the same pathological agent in the donor or in the graft and receptor, sometimes requiring DNA sequencing or special bacterial tip. Replace unreliable surface exchange associated with false positive or false negative results by a sample of the tissue used for the microbiological test would increase the reliability of the results. In most confirmed cases, the same contaminant was identified in the donor or other allografts derived from the same donor and the receiver. This occurred mainly with frozen tissues. Less common were the receptor infections caused by graft contamination during processing. Usually, in these cases, the contaminant was a rare pathogen that was subsequently detected in the banking facilities or selected by antibiotic cocktails used in the procedure []. The transmitted fungal infection was not observed in musculoskeletal alograft, but has been documented for heart valves and arterial grafts. The risk of transmission of donor malignity To our knowledge, there have been no reported cases of transmission of malignities due to musculoskeletal thefts. Exclusion of potential donors with a history of most types of malignancy is required by many guidelines and regulations, although there are differences between the EU and US rules. In a study on the histopathological examination of 1,146 femoral heads recovered from live osteoarthritis patients under hip arthroplasties, Palmer et al. identified two well differentiated lymphomas and a low-grade condrosarcoma in the donated femoral head []. In another study, six out of 504 donated femoral heads that met the criteria for bone transplantation were highly suspicious for low-grade B cell lymphoma []. Two of the six patients mentioned who donated femoral head allografts later developed systemic malignant disease. These conditions were not known at the time of joint replacement and bone donation. It shows that the presence of a malignant tumor at allograft cannot be excluded. The tumors would not have been detected either by reviewing the donor's medical history and a histopathological examination is not routinely part of quality control for recovered bones. It is not known whether the processing or freezing of allografts can destroy malignant cells. However, to date the transmission of malignity has not been reported through the bone alograft transplant. Other adverse reactions Other adverse reactions have been reported allegedly caused by chemicals used for the processing or sterilization of allograft. It was reported that ethylene oxide used for terminal sterilization produces intra-articular reactions with the persistent synovial stroke after implantation of allograft of the bone head []. If bone allografts are preserved with methyl sulfoxide (DMSO), the hypersensitivity of the receiver should be considered as a possibility and reported. As for immune responses, awareness was observed to HLA class I and II antigens after a massive osseo or osteocondral osseo alograft transplant [, , ].Adverse developmentsMechanical properties of bone alografts may be modified by certain processing steps. The surgeon should select the right allograft taking into account the probable modification of the mechanical resistance produced by the different processing techniques and the mechanical requirements of the particular clinical application. For example, a frozen allograft may have a better mechanical resistance than the original bone (±110%) [, , ] while the frozen drying processing will decrease its resistance (30-40%) [, , , , , , ]. The effect of sterilization by gamma irradiation on the mechanical properties of the bone is a function of the dose. The level of 25 kGy, generally accepted for bacterial inactivation, can affect the mechanical properties of soft but not significantly bone tissues [, , , , , , ]. However, the virucidal doses higher than 30 kGy decrease the mechanical strength of the bone [, , , , , , ]. Also depending on the size of the bone graft, revascularization and progressive integration decrease mechanical resistance for several months [, ]. Good communication with the staff of the tissue bank will help the surgeon to select the right type and size of the allograft for their specific purpose, including the expected osteoinductive properties of the demineralized bone matrix allograft [, ].Errors and accidents can occur in the selection of donors, and during the processing of allograft including the packaging and delivery of the tissues, for example, the grafting package. They usually do not cause damage to the receiver. However, these developments may have serious consequences for the recipient and, therefore, when they are discovered, they should be informed that measures can be taken to define and implement corrective measures and lessons can be shared with others on the ground. Debate Although the risk of allograft disease transmission cannot be excluded, its improved safety reflects more precision in donor screening and testing procedures and progress in processing, disinfection and sterilization. Several factors have contributed to improving the safety of allograft: the best performance of serological testing for infectious disease testing [], the selection and selection of donors, the processing protocol used by the bone bank, the guidelines and recommendations of scientific partnerships, national and international regulation and the importance of biovigilance programmes. The selection and selection of donors is now subject, in most countries, to strict regulations prohibiting the recovery of risk donors. With regard to viral contamination, one of the main factors was the evolution of the serological trial of basic antibodies immunosassay to NAT tests, which decreases the period of diagnostic window []. For more than 11 years depending on the virus, contamination has not been reported for musculoskeletal tissues thanks, in part, to the reliability of the tests. The positive influence of the processing protocols developed within the bone bank also appears in the review. Historically, with a possible exception, all cases of viral contamination involved freshly frozen or cryoperated allografts. There are no transmission infections and very few adverse events came from bone aclogation caused by the processed freeze. The quality processes of tissue banks include validation of tissue banking procedures for viral and microbiological decontamination. National and international regulation, such as EU directives, requires the official authorization of the bone bank with periodic regulatory audits. A very important new step to improve the safety of allograft is the additional requirement for an effective bio surveillance program, based on the recognition and notification of adverse results by surgeons. A recent publication by the United States Food and Drug Administration (FDA) shows that more and more adverse incidents associated with tissue transplantation are being reported, as this was mandatory in the United States []. The best way to further improve the safety and quality of musculoskeletal allografts will be to report adverse reactions and events. In this evolution, the surgeon must play an important role in the presentation of such incidents. Complaint and investigation of an incident will prevent many others. After investigating whether the pathogen could have arisen from environmental contamination in the O.R., or was of patient origin, the infection should be reported to the tissue bank with an indication of the imputability of the transplanted allograft and an indication of the severity of the reaction and the long-term consequences. Imputability is an assessment of the likelihood that the negative result in the receiver has been caused by the transplanted allograft and must be evaluated, based on the available information, either: proven, likely, possible, improbable or excluded. Providing accurate diagnosis, the type of pathogen and the exclusion of environmental and hospital causes will be very important to ensure that the tissue bank can focus on its own research. For example, if the suspected contamination by allograft is viral with symptoms that develop weeks or months after surgery, the tissue bank will focus more on research on the eligibility, detection and testing of donors and less on tissue processing failures. The increased recognition of traceability issues has led to the strengthening of professional partnerships by several professional associations and Governments in addition to existing regulations. A unique identification system is required worldwide, and this should be extended through all biological materials: blood, cells, tissues and organs. International associations of cell therapy have agreed on the adoption of standard terminology and the 128 () system of the International Blood Transfusion Society (ISBT) and the application is underway. A joint initiative of international ocular banking associations to develop agreed terminology using ISBT 128 is also at an advanced stage. The banking and organ transplant communities are also in the process of determining how this system could be accepted and implemented []. The normalization of nomenclature and codification systems at the international level will provide institutions with the ability to track allografts of a common donor who may have donated multiple organs and tissues and cause adverse reactions in many patients. The tissue bank considers several elements when investigating a reported infection: the type of alograft processing (fresh, frozen, cryopreserv, freeze-dried), the origin and nature (bone, tendon, skin, vascular) of the graft, review of donor evaluation, including blood analysis, possible retest of blood samples from stored donors and coprocessed tissues and results of environmental monitoring. A tissue bank investigation will determine the cause of pollution and whether standard operational practices were followed, whether there could have been failures in tissue processing, selection or testing of donors and remedial and preventive measures. The tissue bank is responsible for reporting this to the competent authority. The initial assessment, research and reporting by the surgeon are therefore important to improve future medical care. Normally, an orthopedic surgeon tracks the patient after surgery. Often, the surgeon performs a long-term annual follow-up (such as the standard for orthopedic implants, for example, hip arthroplasty). Sometimes, due to distance or other factors, general practitioners and other health care providers are in the care of the patient. Those who provide postoperative follow-up should inform surgeons of any significant illness that arises after surgery. This is an important aspect of a bio surveillance system. Otherwise, you can lose the connection of an infection and a allograft and notify a tissue bank. The NOTIFY project is developing a digital database for adverse events and reactions and developing educational materials for surgeons and other doctors that provide medical care to transplant recipients to help in the recognition of transmitted diseases and other adverse outcomes. Conclusion The quality and safety standards for the donation, acquisition, testing, processing, conservation, storage and distribution of musculoskeletal allografts have improved significantly over the past three decades. Currently, these alografts provide orthopedic surgeons with a useful and safe tool to repair bone defects. In addition, depending on their processing and nature, they provide osteoconductive, osteoinductive and mechanical properties. When all quality and safety requirements are met, adverse events and reactions must be extremely rare. However, adverse events still arise due to human errors, accidents, equipment failure, unexpected tissue defects or new unexpected circumstances such as emerging diseases. Only after the rapid presentation of these exceptional events and reactions, an investigation can begin to analyse the cause and that remedial measures, where possible, can be defined and applied to avoid repetition of events. Of course, an important requirement is to ensure the complete traceability of the applied allograft. To maintain and improve its safe use, WHO launched the NEWS Project in 2010 encouraging all surgeons to inform their bone bank and their national bio surveillance institution of events and serious reactions related to the use of allografts. For orthopedic surgeons working in countries without national agency to receive their case reports, they can download from the SICOT website () a standard report form that can be returned to SICOT. This programme is particularly important at a time when globalization opens the exchange of medical products across national boundaries and when effective bio surveillance is needed to preserve the safety of tissue transplantation. Table 6Standard requisites to deliver safe and reliable musculoskeletal allografts Weaving establishmentsWeather authopedicsComputing of tissue establishments and their activities by the competent authority(s) Weave donors: selection, tests Voluntary donation and informed consent Medical and social history: evaluation using strict selection criteria to exclude those who have infection and malignancyExamination doctorTesting for transmisible infected agents by serological and NAT methods carried out by licenced testing laboratoriesRevision of postmortem autopsy Most have cells, fat, marrow, blood removed through mechanical and chemical means Many have been disinfected; many have been sterilized by gamma irradiation or other methods Septically processed in high quality clean rooms Tissue: quality and safetyThe established and updated quality system: use of validated procedures Quality control (including microbiological tests) Pathogen InactivationTrability, Biovigilance Identification and Tissue LabelingTrability of donor tissues to patient and vice versa, and products and materials that come into contact with the biovigilance tissues: presentation and research of adverse reactions, adverse events ReferencesFormats: Share , 8600 Rockville Pike, Bethesda MD, 20894 USA
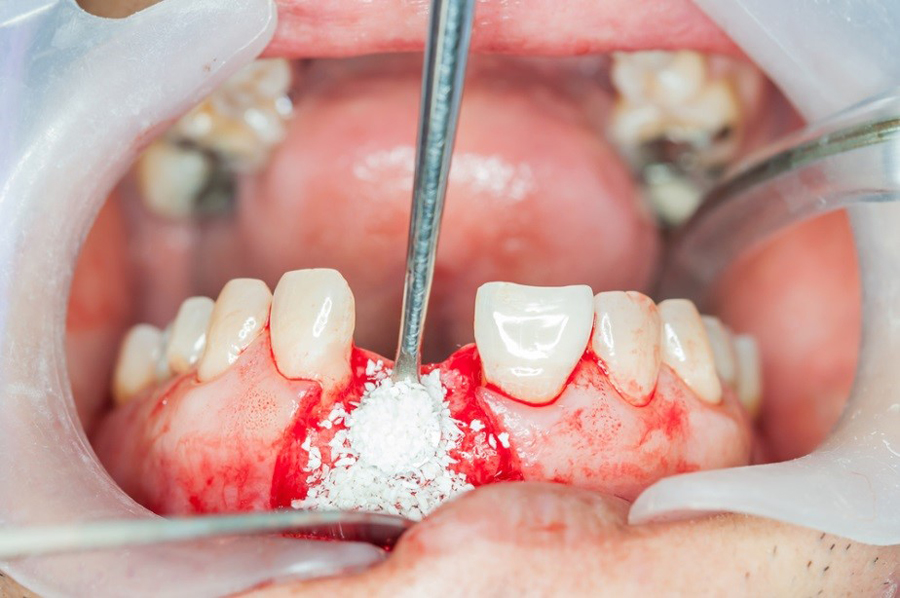
Dental Bone Graft; tips for after care and reduce the complications -
Dental Bone Graft; tips for after care and reduce the complications -
Bone Grafting for Dental Implants - Dentist Athens, AL - Dental Education Library

Can a Dental Bone Graft Get Infected? - Restoration Smiles
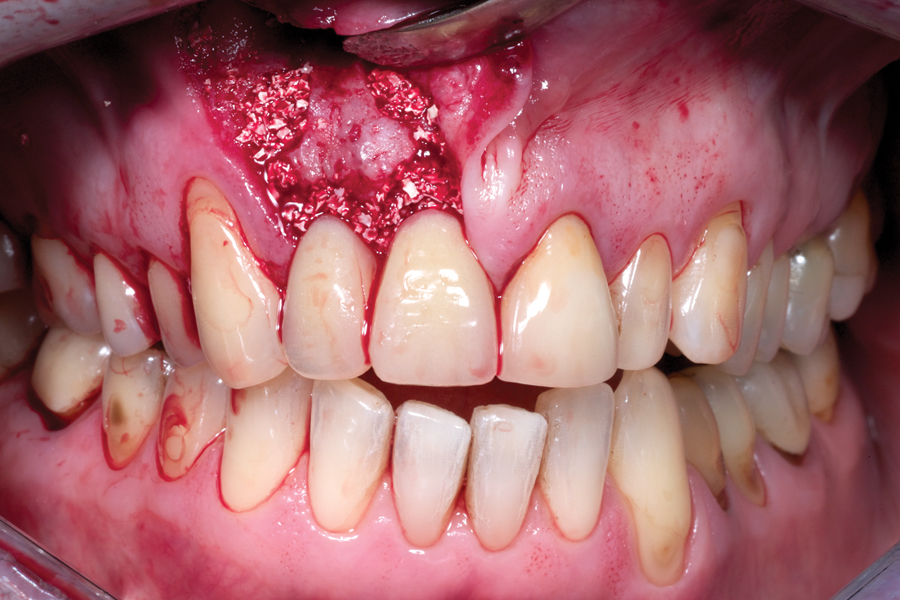
Bone Grafts For Implant Dentistry: The Basics - Oral Health Group
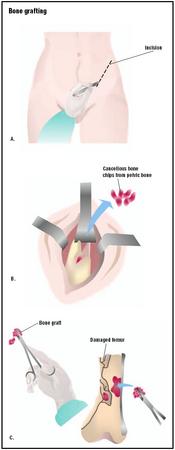
Bone Grafting - procedure, recovery, test, blood, removal, pain, complications, adults
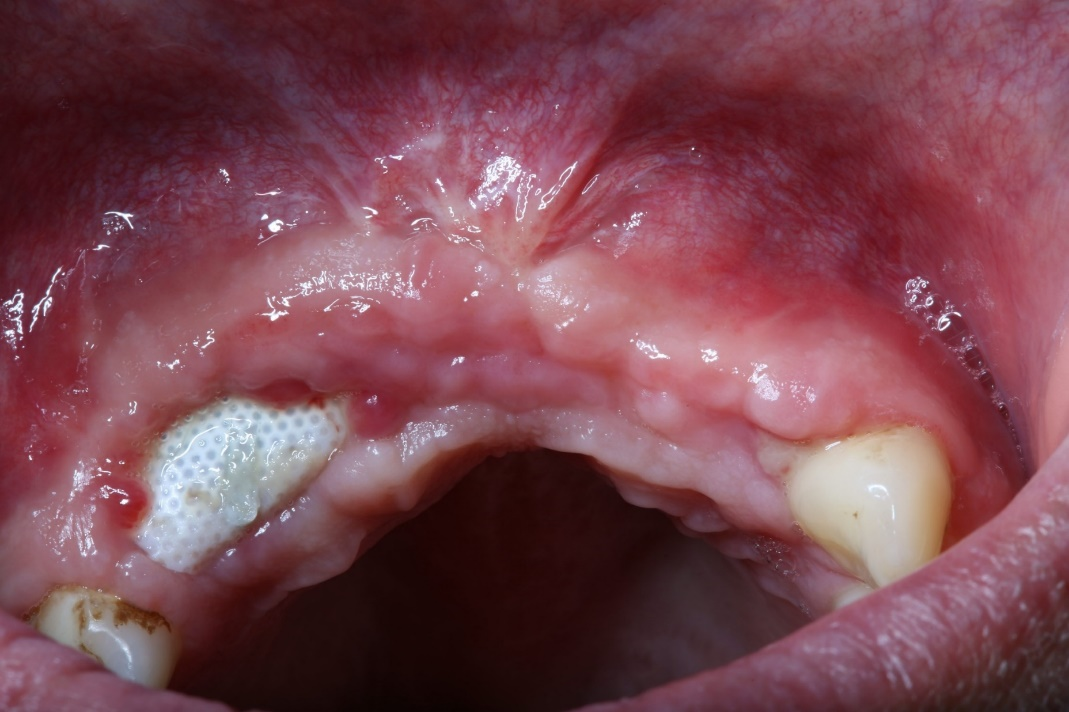
Bone augmentation: Failures and complications | Perio-Implant Advisory
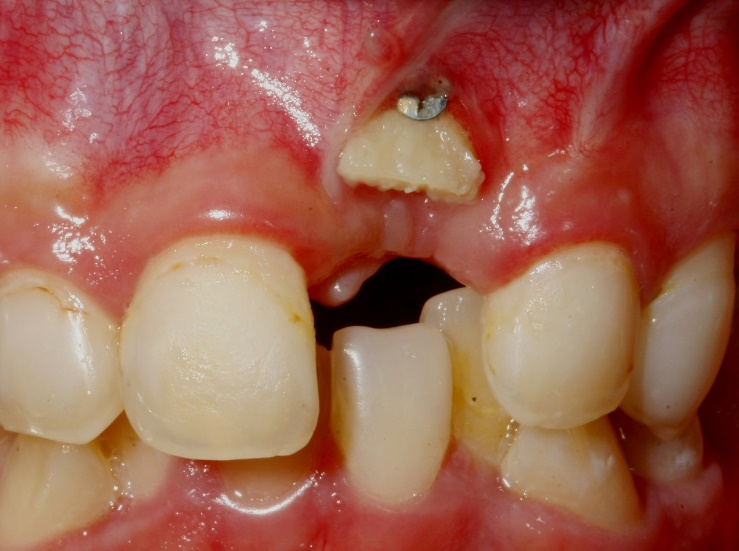
Bone augmentation: Failures and complications | Perio-Implant Advisory

Bone graft rejection symptoms after dental implant | veinscny.com

The Bone is Showing After Extraction Graft! | Ramsey Amin, DDS
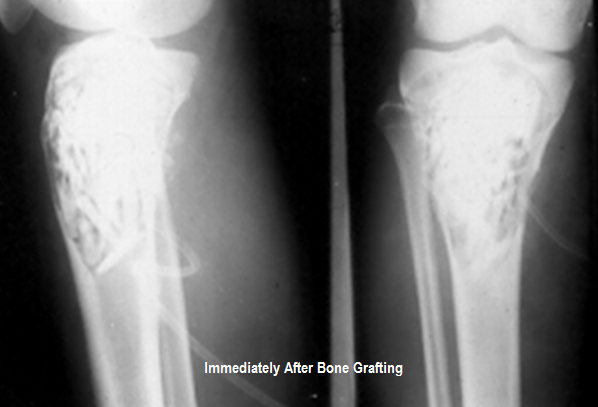
Bone Grafts - Types, Mechanism, Techniques and Complications | Bone and Spine

Bone Graft: Purpose, Procedure, and Risks

Bone Graft in Spinal Fusion Surgery
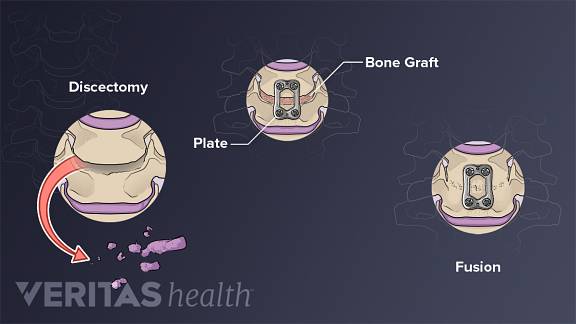
Controversies about Spinal Fusion Surgery: Allografts, Autografts, and Fusion Levels
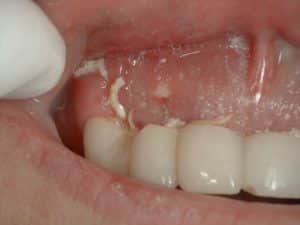
The Bone is Showing After Extraction Graft! | Ramsey Amin, DDS
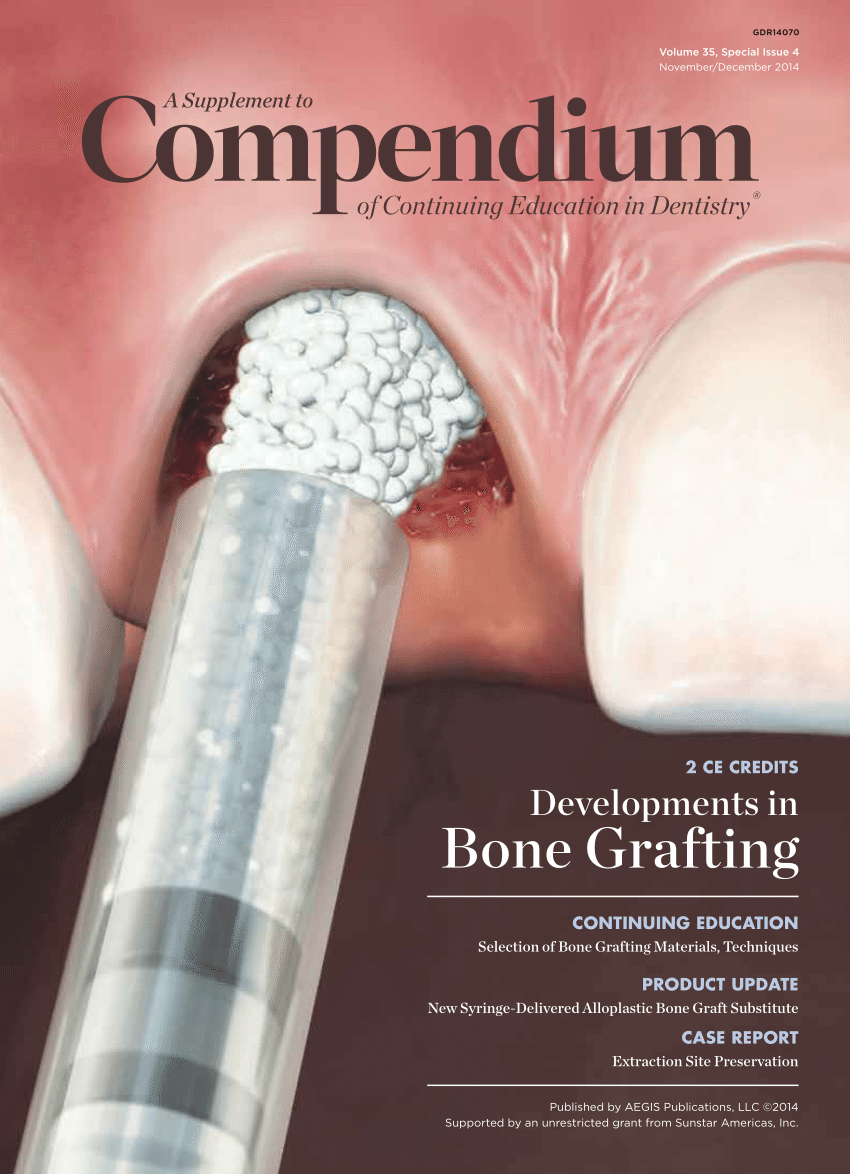
PDF) Bone grafting: History, rationale, and selection of materials and techniques

Bone Graft in Spinal Fusion Surgery

What You Need To Know About A Dental Bone Graft

Bone Grafts: New Developments

Transplant rejection - Wikipedia
Bone Graft: Purpose, Procedure, and Risks

Bone Graft: Purpose, Procedure, and Risks

Solid Growth: Bone Grafts' Role in Spine Surgery and Fusion Success
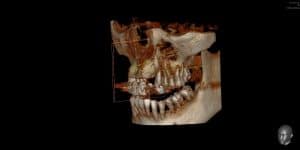
The Bone is Showing After Extraction Graft! | Ramsey Amin, DDS

Bone Grafting | Jackson Oral Surgery | Metairie, LA

Bone Grafts For Implant Dentistry: The Basics - Oral Health Group

Bone Grafting | Johns Hopkins Medicine
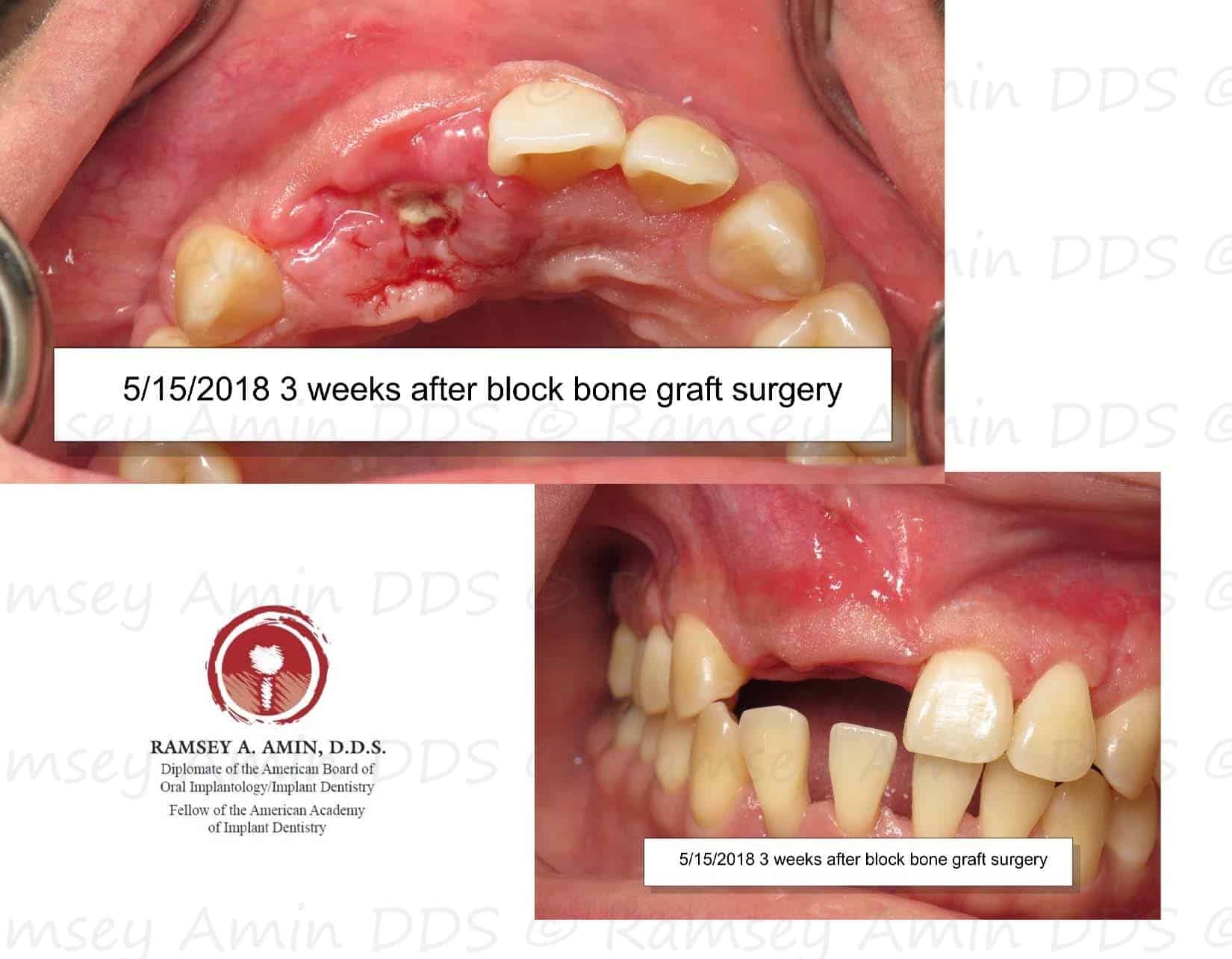
Complication - Bone Graft is Exposed – Platelet PRF Treatment | Ramsey Amin, DDS

Bone Grafting Toronto | The Most Affordable Bone Graft Center in Toronto

Solid Growth: Bone Grafts' Role in Spine Surgery and Fusion Success

Transplant Rejection - an overview | ScienceDirect Topics
Transplant Rejection: Hyperacute, Acute, Chronic & Graft versus Host | Stomp On Step1
Controversies about Spinal Fusion Surgery: Allografts, Autografts, and Fusion Levels

What to Expect From Bone Grafting for Dental Implants

Bone Grafting & Platelet Rich Plasma - Dr. David Mueller | Virginia Facial Surgery

Bone Grafts...How Long Do They Take to Heal? | Ramsey Amin, DDS

Failed or Compromised Skin Graft - Vascular Health Clinics

Causes of late allograft dysfunction a | Download Table

Antibody-Mediated Rejection in Cardiac Transplantation: Emerging Knowledge in Diagnosis and Management | Circulation
Posting Komentar untuk "cadaver bone graft rejection symptoms"